- Author(s): K. Venkatesan, P. V. V. Mohanaraob and V. S. V. Satyanarayana
- Category: HTML FullText
- Published: 02 February 2017
- Views: 4183
Telmisartan - Antihypertensive Drug: A Short Review on Synthetic Methods
K. Venkatesana, P. V. V. Mohanaraob and V. S. V. Satyanarayanac,*
aDepartment of Humanities and Sciences, CVR College of Engineering, Hyderabad, Telangana –501510, India.
bR&D Chemistry, Acebright (India) Pharma Pvt. Ltd., KIADB Indl. Area, Jigani, Bangalore – 560105.
cReaxZen Healthcare Pvt. Ltd., Boduppal, Hyderabad – 500039, India.
Published Online 09 01 2017
Telmisartan is a potent antagonist of the angiotensin II type-1 (AT1) receptor that is used for the treatment of essential hypertension. It selectively inhibits stimulation of the AT1 receptor without affecting other receptor systems involved in cardiovascular system. This review describes the different synthetic routes followed for the total synthesis of Telmisartan which will be useful for the researchers to find several literature reported synthetic methods in one place who intended to develop a new eco-friendly and cost-effective method in industrial scale.
INTRODUCTION
Telmisartan (I) is a potent angiotensin II receptor blocker (ARB) antagonist used in the treatment of essential hypertension and also used to reduce the risk of heart attack, stroke.1-7 It was discovered by Boehringer Ingelheim.8 It has high bioavailability, long half life, good security, few side effects, so it can be used as a first-line drug in the treatment of hypertension. Telmisartan is currently available in the market as an antihypertensive drug under the trade name of MICARDIS.9 Telmisartan is an important member of this class of top-selling drugs because it has the strongest binding affinity to the AT1 receptor, excellent bioavailability, and a once-per-day dosage.10,11
Telmisartan is an angiotensin II receptor blocker shows high affinity for the angiotensin II receptor type 1 (AT1).12 In addition to blocking the rennin-angiotensin system (RAS), which is the central regulator of blood pressure and electrolyte homeostasis.13 In the early 1990s, Merck introduced the nonpeptidic orally active angiotensin II receptor antagonist losartan (Lozaar) as the first member of a new class of antihypertensive drugs called sartans, which contain a characteristic ortho functionalized biaryl moiety.14,15 Telmisartan exhibits favorable effects on renal function in laboratory animals and is not associated with significant ancillary pharmacological effects and limited clinical suggest the potential superiority of telmisartan over losartan 50 mg once daily.16-19 Telmisartan acts as a selective modulator of peroxisome proliferator activated receptor gamma (PPAR-γ), a central regulator of insulin and glucose metabolism. It is believed that telmisartan dual mode of action may provide protective benefits against the vascular and renal damage caused by diabetes and cardiovascular disease (CVD).20-22
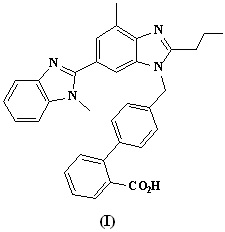
Figure 1. Chemical structure of Telmisartan.
Literature showed that telmisartan tablet reduces the tissue weight of WAT (white adipose tissue) and the liver but does not affect other organs, such as the heart or kidneys.23 This review presents relevant information on the total synthesis of Telmisartan by taking different precursors and using distinct synthetic methodologies.
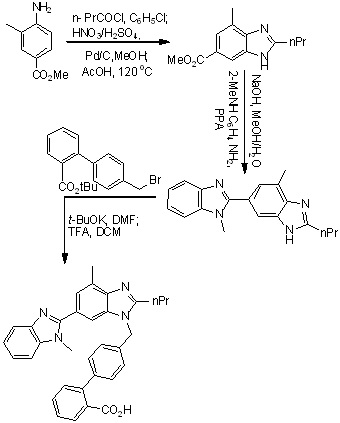
Scheme 1. First reported synthesis of Telmisartan.
SYNTHETIC METHODS
The first total synthesis of telmisartan was reported by Ries et. al., in 1993 (Scheme 1),24 4-amino-3-methylbenzoic acid methyl ester used as an starting material which on undergo acylation with butyryl chloride, followed by nitration using nitrating mixture, reduction of the nitro group using Pd/C and then cyclization of the resulting amine to the benzimidazole derivative using acetic acid. After saponification free carboxyl group present in benzimidazole derivative condensed with N-methyl-1,2-phenylenediamine to form bisbenzimidazole, which is finally alkylated with the 4-(bromomethyl)-2-biphenylcarboxylic acid tert-butyl ester and then hydrolysis to get telmisartan.
In 2007, Srirami Reddy et. al.,25 reported the total synthesis of telmisartan (Scheme 2) from 4-butyrylamino-3-methyl-5-nitro-benzoic acid methyl ester as a starting material due to its commercial availability. First the nitro group was reduced to obtain amine using Pd-C catalyst and the filtrate was concentrated which was directly treated with aqueous sodium hydroxide to furnish benzimidazole intermediate. The intermediate was then condensed with N-methyl-benzene-1,2-diamine in polyphosphoric acid at 150-155 °C to obtain the dibenzimidazole intermediate. The telmisartan was obtained by the alkylation of 4’-Bromomethylbiphenyl-2-carboxylic acid methyl ester with 1,4,-Dimethyl-2’-propyl-1H,3’H-2,5’-dibenzimidazole in acetone in the presence of KOH and the product precipitated was further converted to a hydrochloride salt in methanol. In the next step, hydrochloride salt was hydrolyzed via saponification process using potassium hydroxide as a base in acetonitrile to get the final product - Telmisartan.
In 2008, Lukas et al.,26 reported a new synthetic way for the synthesis of telmisartan by using phthalic anhydride and 3-methyl-4-nitrobenzoic acid represented in Scheme 3. 3-methyl-4-nitrobenzoic acid undergoes reduction in presence of palladium on charcoal followed by acylation of the amino group with butyryl chloride and then nitration to form 4-butyramido-3-methyl-5-nitrobenzoic acid. The condensation of the carboxyl group of resulted nitrobenzoic acid derivative with n-methyl-1,2-phenylenediamine to form the intermediate N-(2-amino-6-methyl-4-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)butyramide.
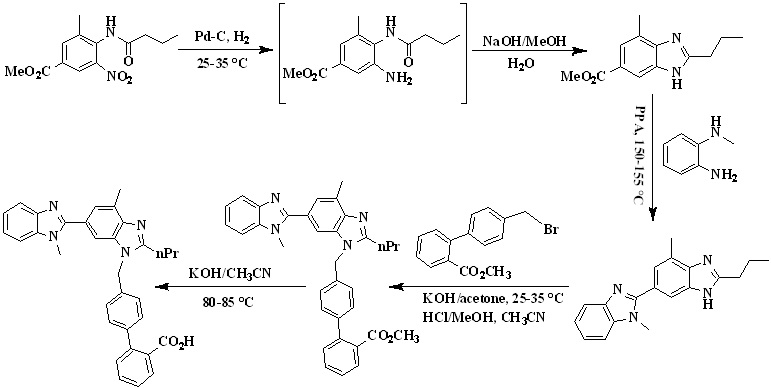
Scheme 2. Synthetic protocol of Telmisartan reported by Srirami Reddy et al.
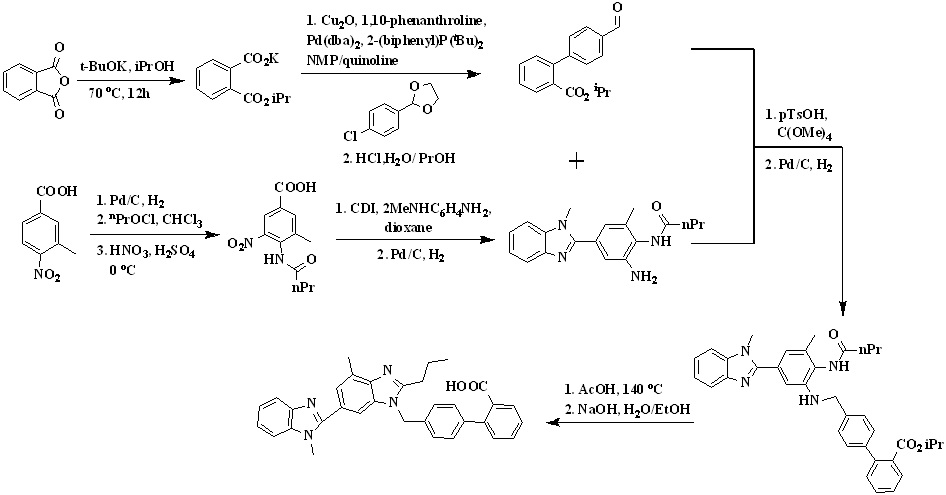
Scheme 3. Synthetic protocol of Telmisartan reported by Lukas et al.
The reductive amination of the biaryl aldehyde reacting with the resulted intermediate was carried out in the presence of p-toluenesulfonic acid/tetramethyl-o-carbonate in toluene, followed by a solvent exchange to isopropyl alcohol and hydrogenation to form compound isopropyl 4'-(((2-butyramido-3-methyl-5-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)amino)methyl)-[1,1'-biphenyl]-2-carboxylate. The amine isopropyl 4'-(((2-butyramido-3-methyl-5-(1-methyl-1H-benzo[d] imidazol-2-yl)phenyl)amino)methyl)-[1,1'-biphenyl]-2-carboxylate was cyclized into the n-propylbenzimidazole in refluxing glacial acetic acid to form telmisartan isopropyl ester was saponified with sodium hydroxide in methanol and water, and subsequent acidification led to the precipitation of telmisartan.
In 2008, Kankan et al.,27 reported an improved process for the preparation of telmisartan (Scheme 4) by the condensation of [lH-Benzimidazole-2-n-propyl-4-methyl-6-(1'-methyl benzimidazole-2'-yl)] and methyl-4(bromomethyl)biphenyl-2-carboxylate in presence of KOH and dimethyl sulphoxide followed by hydrolysis to get the final product.
In 2009, Sanjeev Kumar et al.,28,29 developed an efficient two-stage process employing Suzuki coupling to afford telmisartan represented in Scheme 5. Condensation of 2-(4'-chloromethyl-biphenyl-2-yl)-4,4-dimethyl-4,5-dihydrooxazole with dibenzimidazole derivative to form the key intermediate 3'-[2'-(4,4-Dimethyl-4,5-dihydro-oxazol-2-yl)-biphenyl-4-ylmethyl]-1,7'-dimethyl-2'-propyl-1h,3'h-[2,5'] bibenzoimidazolyl, which is on acid hydrolysis to provide telmisartan in good yield. Chlorination of [2’-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-biphenyl-4-yl]-methanol with thionyl chloride at low temperature afforded the key intermediate 2-(4'-chloromethyl-biphenyl-2-yl)-4,4- dimethyl-4,5-dihydrooxazole.
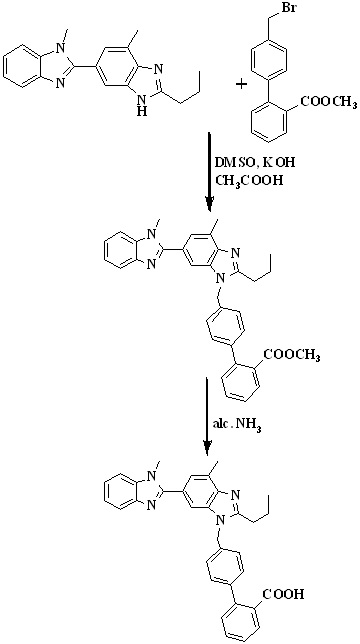
Scheme 4. Synthetic protocol of Telmisartan reported by Kankan et al.
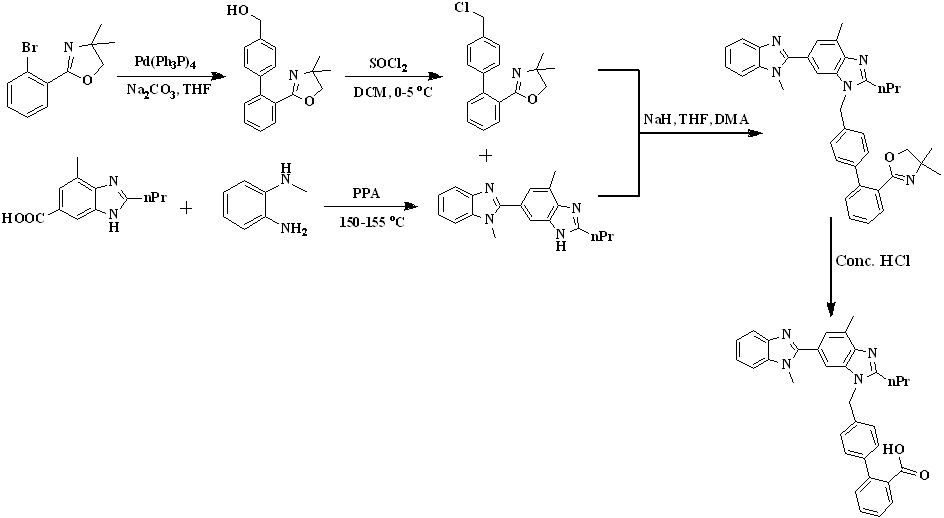
Scheme 5. Synthetic protocol of Telmisartan reported by Sanjeev Kumar et al.
In 2010, Sanjeev Kumar et al., also developed an efficient two-stage process employing Suzuki coupling for the preparation of 2-(4'-chloromethyl-biphenyl-2-yl)-4,4-dimethyl-4,5-dihydrooxazole and later condensation with dibenzimidazole derivative. Finally, acid hydrolysis of intermediate 11 provided telmisartan in good yield (Scheme 6).30 Authors identified 4-(hydroxymethyl) phenylboronic acid and 2-(2-bromophenyl)-4,4-dimethyl-2-oxazoline as key starting materials for the preparation of key intermediate 2-(4'-chloromethyl-biphenyl-2-yl)-4,4-dimethyl-4,5-dihydrooxazole (Scheme 6). Suzuki coupling was employed for the preparation of the key intermediate by the reaction of 2-(2-bromophenyl)-4,4-dimethyl-2-oxazoline with 4-(hydroxymethyl) phenylboronic acid in the presence of aqueous sodium carbonate and tetrakistriphenyl phospine palladium (0), which produced [20-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-biphenyl-4-yl]-methanol in more than 95% yield; with further chlorination using thionyl chloride at low temperature afforded the key intermediate 2-(40-chloromethyl-biphenyl-2-yl)-4,4-dimethyl-4,5-dihydrooxazole in more than 99% yield.
In 2010, Mehta et al.,31 reported an improved synthetic route for the synthesis of telmisartan via Suzuki coupling presented in Scheme 7. The key intermediate, 2’-(4,4-dimethyl-4,5-dihydro-1,3-oxazol-2-yl)biphenyl-4-carbaldehyde was prepared by reacting 2-(2-bromophenyl)-4,4-dimethyl-2-oxazoline with 4-formyl phenylboronic acid in presence of aqueous sodium carbonate and tetrakistriphenylphospine palladium (0).
The intermediate on reduction with sodium borohydride to form [2’-(4,4-dimethyl- 4,5-dihydro-1,3-oxazol-2-yl)biphenyl-4-yl]methanol. The chlorination with thionyl chloride at low temperature afforded the
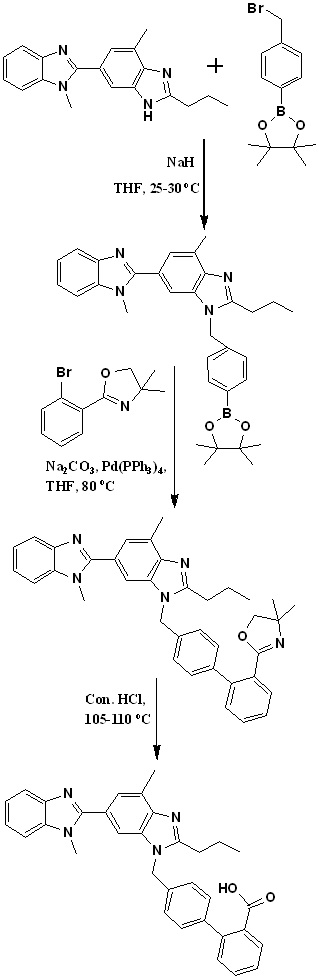
Scheme 6. Synthetic protocol of Telmisartan reported by Sanjeev Kumar et al.
key intermediate 2-[4'-(chloromethyl)biphenyl-2-yl]-4,4-dimethyl-4,5-dihydro-1,3-oxazole. 1,4'-dimethyl-2'-propyl-1H,3'H-2,5-dibenzimidazole react with key intermediate in presence of sodium hydride and hydrolysis to form telmisartan.
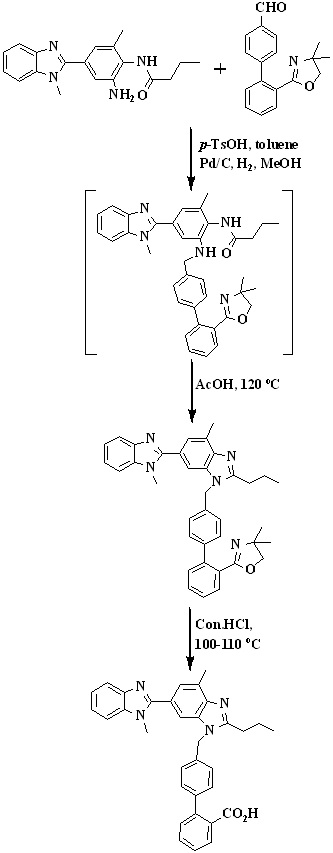
Scheme 7. Synthetic protocol of Telmisartan reported by Mehta et al.
In 2010, Nageswar Rao et al.,32 developed a facile, cost-effective, and improved one-pot synthesis for telmisartan described in Scheme 8. They have chosen 1,4'-dimethyl-2'-propyl-1H,3'H-2,5'-dibenzimidazole as a starting material for the preparation of 4’-(1,4’-dimethyl-2’-propyl-1H-[2,5’]bibenzimidazolyl-3’-ylmethyl)biphenyl-2-carboxylic acid methyl ester, because of its commercial availability. Condensation of compound a with compound b in acetonitrile using potassium hydroxide as a base to afford c, which is an in situ, and then converted into telmisartan by adding potassium hydroxide lot wise instead of dumping to avoid impurity formation.
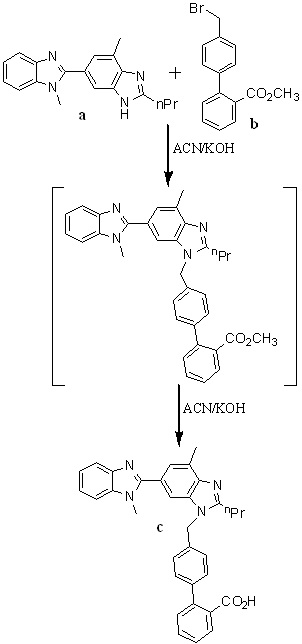
Scheme 8. Synthetic protocol of Telmisartan reported by Nageswar Rao et al.
In 2011, Srinivas et al.,33 reported the synthesis of telmisartan by using 4-formylphenylboronic acid and 2-(2-bromophenyl)-4,4-dimethyl-2-oxazoline used as the starting material detailed in Scheme 9. Thus, Suzuki coupling of 2-(2-bromophenyl)-4,4-dimethyl-2-oxazoline with 4-formyl phenyl boronic acid in presence of aqueous sodium carbonate and
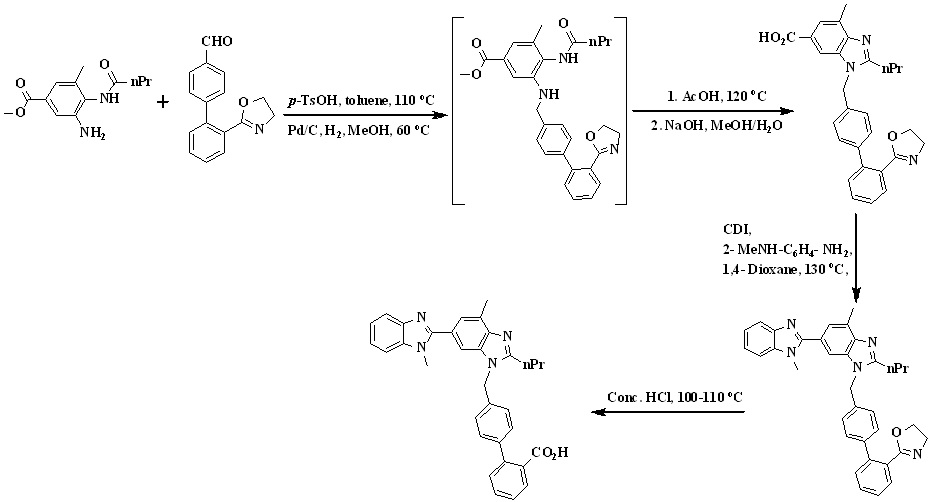
Scheme 9. Synthetic protocol of Telmisartan reported by Srinivas et al.
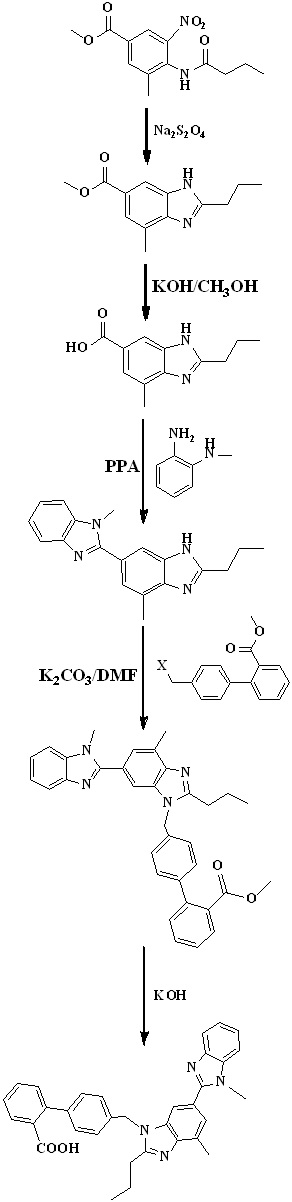
Scheme 10. Synthetic protocol of Telmisartan reported by Ping Wang et al.
tetrakis(triphenylphosphine)palladium(0) in THF gave 2-(4,4-dimethyl-4,5-dihydro-1,3-oxazol-2-yl)biphenyl-4-carbaldehyde. The reaction of biaryl aldehyde with amine was carried out in the presence of p-toluenesulfonic acid in toluene to form the corresponding imine, which on hydrogenation in methanol yielded the amine.
The amine was cyclized in situ to the n-propyl benzimidazole in refluxing glacial acetic acid followed by in situ hydrolysis to the acid to form1-((2'- (4, 4- dimethyl -4, 5-dihydro oxazol -2- yl) biphenyl -4- yl) methyl) -4- methyl-2-propyl-1Hbenzo[d]-imidazole-6-carboxylicacid which is reacting with 1,1’-carbonyldiimidazole (CDI) followed by coupling with N-methyl-1,2-phenylenediamine and in situ cyclization in 1,4-dioxane, afforded the bisbenzimidazole derivative. Finally oxazoline intermediate was treated with concentrated hydrochloric acid to afford telmisartan.
In 2012, Ping Wang et al.,34 reported the total synthesis of telmisartan detailed in Scheme 10. The starting material, o-cresol was treated with chloroform in 10% sodium hydroxide solution to get aldehyde and then nitration via reacting with fuming nitric acid. The phenolic hydroxyl group of compound was protected with dimethyl sulfate followed by reaction with o-phenylenediamine in presence hyderogen peroxide to get compound. The bis-benzimidazole was prepared from benzimidazole substituted o-nitroaniline using butyl aldehyde in the presence of sodium dithionite in the mixture of methanol and water at reflux condition. After this N-alkylation of bis-benzimidazole was done by treated with methyl 4'-(bromomethyl)-2-biphenylcarboxylate in presence of sodium hydride and then saponified with 10% aqueous sodium hydroxide solution in methanol to get telmisartan.
In 2012, Chakrapani et al.,35 reported the low cost synthesis of telmisartan (Scheme 11) by using methyl-4-(butyramido)-3-methyl-5-nitrobenzoate is an starting material undergo reduction using sodium dithionate and cyclisation followed by hydrolysis to form 2-n-propyl-4-mmethyl-6-carboxy benzimidazole. N-methy-o-phenylenediamine on reaction with carboxy benzimidazole derivative in presence of PPA to form bis benzimidazole intermediate. Reaction of 4’-halomethylbipheny-2-carboxylic ester and intermediate in basic condition, followed by hydrolysis to form telmisartan.
In 2013, Premchand et al.,36 were developed cost-effective and improved one-pot synthesis for telmisartan which is detailed given in Scheme 12. The preparation of 4'-((1,4'- dimethyl-2'-propyl(2,6'-bi-1h-benzimidazole)-1'-yl)methyl)-1,1'-biphenyl-2-carbonitrile was done by using 4’-(bromomethyl) biphenyl-2-carbonitrile used as an raw material because it is very cheap and commercially available in the market. Condensation of 2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2′-yl) benzimidazole 2 with 4'-(bromomethyl) biphenyl-2-carbonitrile in acetone using potassium hydroxide to get 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1hbenzimidazole)-1'-yl)methyl)-1,1'-biphenyl-2- carbonitrile followed by hydrolysis using potassium hydroxide in ethylene glycol to get telmisartan.
In 2015, Alex et al.,37,38 reported the total synthesis of telmisartan via Suzuki Cross-Coupling reaction between two functionalized benzimidazoles represented in Scheme 13.
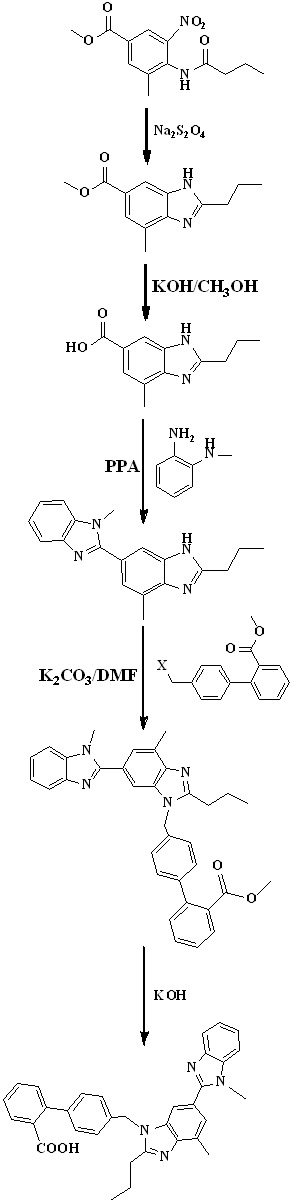
Scheme 11. Synthetic protocol of Telmisartan reported by Chakrapani et al.
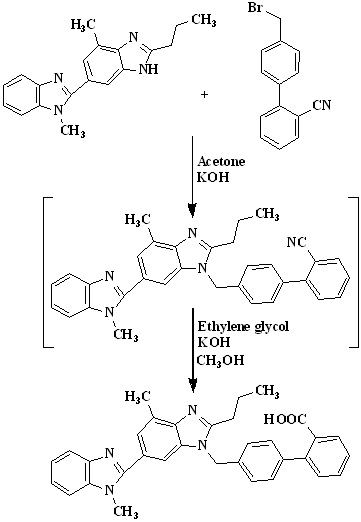
Scheme 12. Synthetic protocol of Telmisartan reported by Premchand et al.
Benzimidazole was first converted in to trifluoroborate salt in two steps. The reaction of 6-bromo-4-methyl-2-propylbenzimidazole and diboron at 100 °C for 5 h in the presence of PdCl2dppf led to the formation of the desired boronic acid pinacol ester and then converted this pinacol boronate directly to the corresponding trifluoroborate salt, as more reactive toward Suzuki cross-coupling reactions. Suzuki cross-coupling of potassium (1-(2'-Carboxy-[1,1'-biphenyl]-4-yl)-4-methyl-2-propyl-benzimidazole-6-yl)trifluoro borate with 2-bromo-1-methylbenzimidazole was carried out under reaction conditions using PdCl2dppf with KOH in a H2O/EtOH solvent system to afford afforded telmisartan.
The spectral characterization data of Telmisartan reported by different authors is given in Table 1.
Properties of Telmisartan:39-41
|
Chemical Formula : |
C33H30N4O2 |
|
Molecular Weight : |
514.6169 g/mol |
|
Melting Point : |
261-263 °C |
|
Water Solubility : |
Insoluble |
|
DMSO Solubility : |
>5 mg/mL at 60 °C |
|
LogP : |
7.7 |
|
Density : |
1.16 |
|
Colour : |
White to slightly yellowish solid |
|
Formal Charge : |
0 |
|
Hydrogen Bond Donar Count : |
1 |
|
Hydrogen Bond Acceptor Count : |
4 |
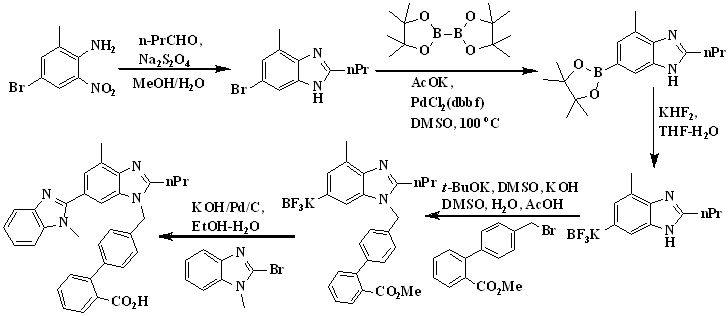
Scheme 13. Synthetic protocol of Telmisartan reported by Alex et al.
Table 1: Characterization data of Telmisartan (I).
|
Author |
m.p. (oC) |
1H NMR |
13C NMR |
Mass (m/z) |
|
Ping Wang34 |
261–262 |
1H NMR (DMSO-d6, 400 MHz, ppm) δ 1.15 (t, J 7.2 Hz, 3H, CH3), 1.94-2.03 (m, 2H, CH2), 2.69 (s, 3H, ArCH3), 3.12 (t, J 8 Hz, 2H, CH2), 3.37 (s, 3H, CH3), 5.40 (s, 2H, CH2N), 6.97 (s, 1H, ArH), 7.05 (s, 1H, ArH), 7.16-7.18 (m, 2H, ArH), 7.33-7.50 (m, 8H, ArH), 8.01-8.03 (m, 1H, ArH), 8.37-8.38 (m, 1H, ArH); |
13C NMR (DMSO-d6, 100MHz, ppm) δ 14.1, 17.0, 22.4, 30.0, 31.8, 48.8, 109.4, 111.3, 119.8, 121.8, 123.1, 123.2, 123.6, 127.1, 127.4, 128.8, 128.9, 129.4, 130.2, 130.4, 133.7, 134.0, 134.6, 135.6, 141.1, 141.8, 142.8, 143.6, 154.0, 156.5, 171.1 |
515.2452 |
|
A. Sanjeev Kumar28-31 |
260–262 |
1H NMR (CDCl3, 400 MHz, ppm) δ 12.8 (1H, s, COOH), 8.42 (1H, d, J 8.0 Hz, ArH), 8.02 (1H, d, J 8.0 Hz, ArH), 7.52-7.28 (8H, m, ArH), 7.20 (2H, d, J 8.0 Hz, ArH), 7.05 (1H, s, ArH), 6.96 (1H, s, ArH), 5.42 (2H, s, - CH2), 3.82 (3H, s, CH3), 2.97 (2H, t, J 7.6 Hz, CH2), 2.74 (3H, s, CH3), 1.92 (2H, m, J 7.6 Hz, CH2), 1.04 (3H, t, J 7.6 Hz, CH3); |
13C NMR (DMSO-d6, 400 MHz, ppm) δ 13.5, 16.7, 20.6, 27.6, 32.7, 47.1, 51.7, 112.0, 112.7, 114.7, 118.6, 125.3, 125.7, 125.8, 127.0, 127.4, 128.6, 129.3, 130.4, 130.6, 131.5, 132.3, 133.1, 133.2, 133.7, 134.5, 140.2, 140.5, 150.2, 157.3, 168.1 |
515 |
|
Lukas J26 |
261–263 |
1H-NMR (CDCl3, 600 MHz ppm) δ 8.36 (d, J 7.7 Hz, 1H), 8.01 (d, J 7.7 Hz, 1H), 7.49 (t, J 7.3 Hz, 1H), 7.43 (t, J 7.4 Hz, 1 H), 7.38 (d, J 7.2 Hz, 1H), 7.28-7.34 (m, 5H), 7.15 (d, J 7.7 Hz, 2H), 7.04 (s, 1H), 6.97 (s, 1H), 5.38 (s, 2H), 3.71 (s, 3H), 3.10 (t, J 7.7 Hz, 2H), 2.68 (s, 3H), 1.96 (sext, J 7.7 Hz, 2H), 1.13 (t, J 7.3 Hz, 3H) |
13C NMR (CDCl3, 151 MHz, ppm) δ 171.1, 156.4, 153.9, 143.5, 142.7, 141.7, 141.0, 135.5, 134.5, 133.9, 133.7, 130.4, 130.2, 129.3, 128.9, 128.8, 127.4, 127.0, 123.5, 123.1, 123.1, 121.8, 119.7, 111.2, 109.4, 48.7, 31.7, 30.0, 22.4, 16.9, 14.1 |
513.2
|
|
Srinivas Ambati33 |
260–262 |
1H NMR (CDCl3,400 MHz, ppm) δ 12.8 (1H, s, COOH), 8.42 (1H, d, J 8.0 Hz, ArH), 8.02 (1H, d, J 8.0 Hz, ArH), 7.52-7.28 (8H, m, ArH), 7.20 (2H, d, J 8.0 Hz, ArH), 7.05 (1H, s, ArH), 6.96 (1H, s, ArH), 5.42 (2H, s, CH2), 3.82 (3H, s, CH3), 2.97 (2H, t, J 7.6 Hz, CH2), 2.74 (3H, s, CH3), 1.92 (2H, m, J 7.6 Hz, CH2), 1.04 (3H, t, J 7.6 Hz, CH3) |
13C NMR (DMSO-d6,100 MHz, ppm) δ 13.5, 16.7, 20.6, 27.6, 32.7, 47.1, 51.7, 112.0, 112.7, 114.7, 118.6, 125.3, 125.7, 125.8, 127.0, 127.4, 128.6, 129.3, 130.4, 130.6, 131.5, 132.3, 133.1, 133.2, 133.7, 134.5, 140.2, 140.5, 150.2, 157.3, 168.1.
|
515 |
|
Alex D37,38 |
---- |
1H NMR (CDCl3, 400 MHz, ppm) δ 8.41 (d, 1H), 8.04 (d, 1H), 7.00-7.52 (m, 12H), 5.43 (s, 2H), 3.76 (s, 3H), 3.16 (t, 2H), 2.73 (s, 2H), 2.02 (m, 2H), 1.18 (t, 3H)
|
13C NMR (CDCl3, 400 MHz, ppm) δ 172.9, 158.2, 155.7, 145.2, 144.5, 143.3, 142.7, 137.2, 136.2, 135.6, 135.3, 132.1, 131.9, 131.0, 130.6, 130.4, 129.0, 128.8, 125.2, 124.9, 124.8, 123.5, 121.4, 113.0, 111.0, 50.5, 33.5, 31.7, 24.1, 18.6, 15.8 |
515.2468 |
|
Srirami Reddy25 |
---- |
1H NMR (CDCl3, 400MHz, ppm) δ 12.8 (s, 1H), 7.05-7.5 (m, 14H), 5.60 (s, 2H), 3.82 (s, 3H), 2.97 (t, J ) 7.5, 2H), 2.63 (s, 3H), 1.88 (q, J ) 7.3, 2H), 1.04 (t, J ) 7.3, 3H); |
13C NMR (DMSO-d6 400 MHz, ppm) δ 13.5, 16.7, 20.6, 27.6, 32.7, 47.1, 51.7, 112.0, 112.7, 114.7, 118.6, 125.3, 125.7, 125.8, 127.0, 127.4, 128.6, 129.3, 130.4, 130.6, 131.5, 132.3, 133.1, 133.7, 134.5, 140.2, 140.5, 150.2, 157.3, 168.1. |
515 |
|
Challa Nageswar Rao32 |
260–262 |
1H NMR (CDCl3, 400 MHz, ppm) δ 12.6 (s, 1H), 7.05-7.5 (m, 14H), 5.86 (s, 2H), 4.0 (s, 3H), 2.5 (t, 2H), 3.08 (s, 3H), 1.84 (q, 2H), 0.99 (t, 3H). |
|
515 |
|
Premchand36 |
260–262 |
1H NMR (DMSO-d6, 400 MHz, ppm) δ 0.98-1.03 (t,3H), 1.73- 1.86 (m, 2H), 2.5 - 2.63 (s, 3H), 2.90-2.95 (s, 2H),3.82 (s, 3H), 5.62 (s, 2H), 7.16-7.34 (m,7H), 7.40-7.59 (m,4H), 7.68-7.70 (m, 3H), 12.86 (s, 1H). |
|
515.50 |
Conclusions
Telmisartan is a potent, insurmountable AII receptor antagonist that shows high selectivity for the AT1 receptor subtype. The present study has been particularly focused a better understanding on different synthetic routes adopted for the preparation of telmisartan scaffold. This article tells about the various cost effective and yield improved methodologies for the total synthesis of Telmisartan.
Acknowledgements
The authors acknowledges the IIT Mandi, Himachal Pradesh for providing necessary facilities.
Notes and References
*Corresponding Author Details: V. S. V. Satyanarayana, ReaxZen Healthcare Pvt. Ltd., Boduppal, Hyderabad 500039, India.
E-mail id.: This email address is being protected from spambots. You need JavaScript enabled to view it.
† Supporting Information (SI): Not available.
1. W. Wienen, N. Hauel, J. C. A. Van Meel, B. Narr, U. Ries and M. Entzeroth, Brit. J. Pharmacol. 1993, 110, 245.
2. K. J. McClellan and A. Markham, Drugs, 1998, 56, 1039.
3. A. J. Battershill and L. J. Scott, Drugs, 2006, 66, 51−83.
4. M. Schupp, J. Janke, R. Clasen, T. Unger and U. Kintscher, Circulation, 2004, 109, 2054.
5. S. C. Benson, H. A. Pershadsingh, C. I. Ho, A. Chittiboyina, P. Desai, M. Pravenec, N. Qi, J. Wang, M. A. Avery and T. W. Kurtz, Hypertension, 2004, 43, 993.
6. M. Miura, S. Satoh, H. Kagaya, M. Saito, T. Inoue, T. Ohkubo, T. Habuchi and T. Suzuki, J. Clin. Pharm. Ther., 2009, 34, 683.
7. T. W. Kurtz, Acta Diabetol., 2005, 42, S9.
8. http://www.boehringer-ingelheim.com/corporate_profile/ history/history3.html
9. http://www.rxlist.com/cgi/generic2/telmisartan.htm.
10. H. Kakuta, K. Sudoh, M. Sasamata and S. Yamagishi, Int. J. Clin. Pharmacol. Res., 2005, 25, 41.
11. W. Kirch, B. Horn and J. Schweizer, Eur. J. Clin. Invest., 2001, 31, 698.
12. M. A. Ondetti and D. W. Cushman, J. Med. Chem., 1981, 24, 355.
13. G. Berellini, G. Cruciani and R. Mannhold, J. Med. Chem., 2005, 48, 4389.
14. P. C. Wong, T. B. Barnes, A. T. Chiu, D. D. Christ, J. V. Dunica, W. F. Herblin and P. B. M. W. M. Timmermans, CardioVasc. Drug ReV. 1991, 9, 317.
15. D. J. Carini and J. V. Duncia, Eur. Pat. Appl. EP0253310A2, 1998.
16. W. Wienen, M. Entzeroth, J. C. A. van Meel, J. Stangier, U. Busch, T. Ebner, J. Schmid, H. Lehmann, K. Matzek, J. Kempthorne-Rawson, V. Gladigau and N. H. Haul, Cardiovasc. Drug Rev., 2000, 18, 127.
17. D. Sylvia, R. H. Beoger, J. Weiss and R. A. Benndorf, Expert Opin. Drug Metab. Toxicol., 2010, 6, 863.
18. R. A. Benndorf, T. Rudolph, D. Appel, E. Schwedhelm, R. Maas, F. Schulze, E. Silberhorn and R. H. Bfger, Metabolism- Clinical and Experimental., 2006, 55, 1159.
19. J. M. Mallion, J. P. Siche and Y. Lacourcière, Hypertension, 1999, 13, 657.
20. R. M. Salama, M. F. Schaalan, M. E. Ibrahim, A. E. Khalifa and A. A. Elkoussi, Open Journal of Endocrine and Metabolic Diseases, 2013, 3, 186.
21. D. Ganesh, S. Anoop, H. Mrunalini, M. Mohini, H. Sachin and S. Abhijit, Int. J. Pharmacol. Res., 2015, 5, 80.
22. D. Fukuda, S. Enomoto, Y. Hirata, R. Nagai and M. Sata Biomed. Pharmacother., 2010, 64, 712.
23. K. Araki, T. Masaki, I. Katsuragi, K. Tanaka, T. Kakuma and H. Yoshimatsu, Hypertension, 2006, 48, 51.
24. U. J. Ries, G. Mihm, B. Narr, K. M. Hasselbach, H. Wittneben, M. Entzeroth, J. C. A. Van Meel, W. Wienen, N. H. Hauel, J. Med. Chem., 1993, 36, 4040.
25. K. S. Reddy, N. Srinivasan, C. R. Reddy, K. Naveenkumar, Y. A. S. Venkatraman, A. Bhattacharya and T. M. Vijayavitthal, Org. Process Res. Dev., 2007, 11, 81.
26. J. Lukas and K. Thomas, J. Org. Chem., 2008, 73, 8631.
27. K. R. Narayanrao, D. R. Rao, P. L. Srinivas and P. Ravikumar, US Patent US 2008/0015359 A1, 2008.
28. A. S. Kumar, S. Ghosh, G. N. Mehta, R. Soundararajan, P. S. R. Sarma and K. Bhima. Synthetic Commun., 2009, 39, 4149.
29. A. S. Kumar, S. Ghosh, R. Soundararajan and G. N. Mehta, ARKIVOC, 2009, (x), 247.
30. A. S. Kumar, S. Ghosh and G. N. Mehta, Arch. Appl. Sci. Res., 2010, 2, 135.
31. A. S. Kumar, S. Ghosh and G. N. Mehta, Beilstein J. Org. Chem., 2010, 6, 25.
32. C. N. Rao, T. Naresh, K. Satyanarayana, B. R. Reddy and G. M. Reddy, Synthetic Commun., 2010, 40, 530.
33. A. Srinivas, P. H. Rao, T. V. Maruthikumar, N. Sreenivasan, A. N. Babu, Der. Chemica. Sinica., 2011, 2, 151.
34. P. Wang, Z. Guo-jun, W. Ya-ping, W. Xiang-jing, W. He-geng and X. Wen-sheng, Tetrahedron, 2012, 68, 2509.
35. L. Chakrapani, K. N. Raju, K. Sridhar, M. S. P. Sarma and B. N. Choudary, WO Patent WO2012/028925 A2, 2012.
36. B. P. Premchand, P. Anand, B. S. Devanand and B. R. Chaudhari, Int. J. Res. Pharm. Biomed. Sci., 2013, 4, 293.
37. D. M. Alex, R. S. Ali, B. Katherine and B. F. Gupton, J. Org. Chem., 2015, 80, 1915.
38. D. M. Alex, R. S. Ali, B. Katherine and B. F. Gupton, J. Flow. Chem., 2015, 5, 145.
39. https://pubchem.ncbi.nlm.nih.gov/compound/Telmisartan #section=UNII
40. http://www.drugbank.ca/drugs/DB00966
41. http://www.chemicalbook.com/ChemicalProductProperty _EN_CB4266172.htm
