- Author(s): Anand Sivadas, Noormohamed Abdulmalik and Narayanan Subbaraya
- Category: HTML FullText
- Published: 02 February 2017
- Views: 1622
Novel Thienyl Acrylates Bearing Different Heterocyclic Moieties and Their Larvicidal Activity
Anand Sivadasa,* Noormohamed Abdulmalika and Narayanan Subbarayab
aDepartment of Synthetic Chemistry, Orchid Chemicals and Pharmaceutical Ltd., Research and Development, Shollinganallur, Chennai, Tamilnadu, India.
bDepartment of Chemistry, Presidency College, Chennai, Tamil Nadu, India.
Published Online 16 01 2017
Several substituted thienyl acrylates have been prepared from substituted thiophene-2-carboxaldehyde, methyl acrylate and various hydroxyl heterocyclic compounds via Baylis-Hillman reaction. Structures of the synthesized thienyl acrylates were confirmed by the spectral characterization using analytical methods FT-IR, 1H NMR, 13C NMR and mass. A particular series of thienyl acrylates containing different quinoline heterocyclic moieties were examined for their Larvicidal activity against the second and fourth instar larvae of Culex quinquefasciatus and Aedes aegypti.
Heterocycles constitute one of the most important classes of organic compounds and are distributed over a great extent in nature. A range of important activities are associated with this class of substances. Most of the today’s synthetic drugs as well as many known natural drugs contain heterocyclic residues. About half of the known organic compounds have structures that incorporate at least one heterocyclic component. Heterocycles involving nitrogen, oxygen and sulfur as hetero atoms are common and more thoroughly studied than those involving other elements like phosphorus, boron, tin and silicon. Some of the natural amino acids, many essential vitamins (vitamin B series and vitamin C), alkaloids, antibiotics and dyes contain heterocyclic ring components.1-14 In addition to the predominant applications in biological sector, their potential is substantially utilized in synthetic chemistry as well as in material science. Development of new synthetic strategies for heterocycles construction became an area of great interest as a result of the phenomenal potential possessed by heterocycles.
Sulfur containing heterocycles are of immense importance biologically and industrially.15 The thiophene is the oldest known organic sulfur-containing heterocycles that possess the only one ‘S’ atom in a five membered ring. In the literature, there are many anti-cancer and anti-inflammatory agents that possess the thiophene heterocycle.16-18 Many of them are being used in the clinic and some are undergoing clinical trials.
OSI 930 (3-[(quinolin-4-ylmethyl)-amino]-thiophene-2-carboxylic acid (4-trifluoromethoxy-phenyl)-amide) is a substituted thiophene derivative which is an orally active inhibitor of two clinically validated targets: c-Kit and the vascular endothelial growth factor receptor-2 (VEGFR-2). OSI-930 is designed to target both cancer cell proliferation and blood vessel growth (angiogenesis) in selected tumors.19,20 Tiaprofenic acid and Suprofen are substituted thiophene derivatives with anti-inflammatory activity. Both the drugs are nonsteroidal anti-inflammatory drugs, prescribed mostly for the treatment of musculoskeletal pain, including back pain, osteoarthritis and rheumatoid arthritis.21,22
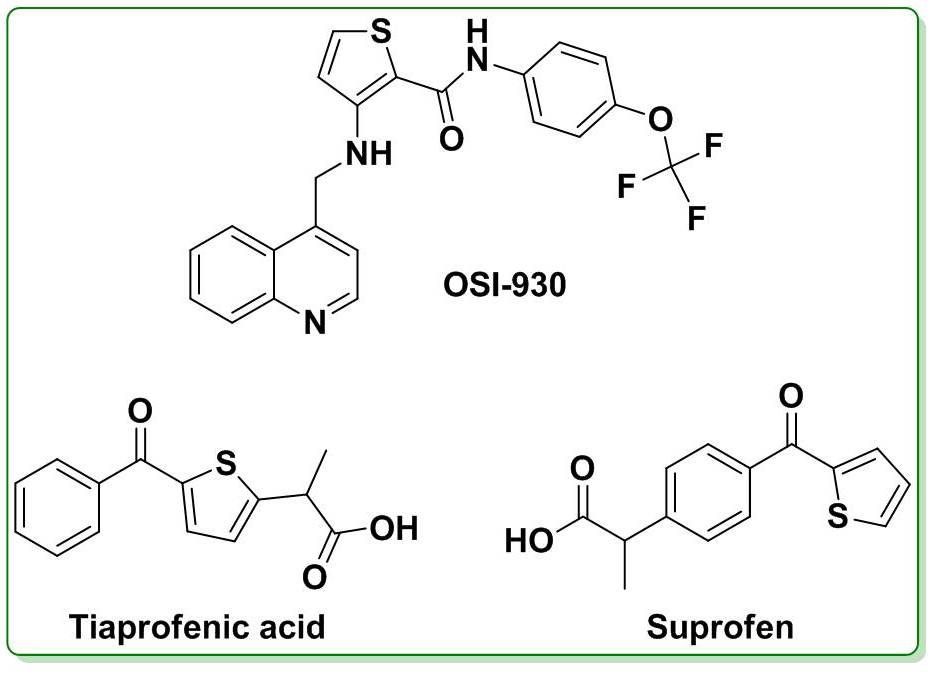
Figure 1. Chemical structures of thiophene based drugs.
From the examples listed above, it is evident that the thiophene heterocycle is an excellent scaffold for anti-cancer and anti-inflammatory drug design and variation
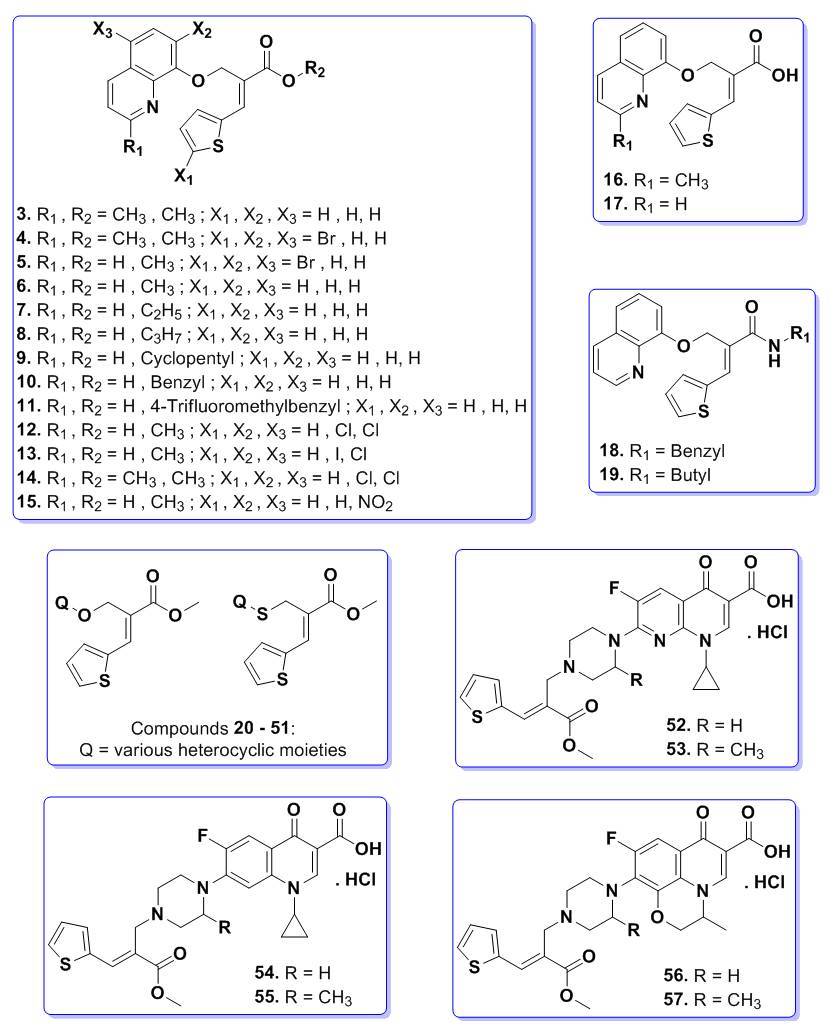
Figure 2. Different synthesized thienyl acrylate derivatives.
of substituents around this thiophene heterocycle may alter the mechanism of the activity action. Besides the substituted thiophenes, intensive research has been carried out on thiophene containing other heterocycles in the search for anti-malarial lead compounds. Recent reports have shown that a number of substituted thiophenes containing different heterocyclic moieties also possess anti-malarial activity. Some of these compounds include derivatives of quinoline containing substituted thiophenes.23
Hence, it would be rational to explore the fusion of thiophene ring with other heterocycles in an attempt to discover new thiophene scaffolds which may possess significant biological properties. With this as the aim, and in continuation of the efforts of our research group to find potent anti-malarial leads, the interest was extended to explore the synthesis of quinoline containing thiophene system and its analogues.
Also the nitrogen heterocycles present in the natural and synthetic drugs are very frequently involved as key constituents in biological processes. Particularly, Quinolines represent the major class of heterocycles, and a number of preparations have been known since the late 1800’s. The quinoline ring system occurs in various natural products, especially in alkaloids. The quinoline skeleton is often used for the design of many synthetic compounds with diverse pharmacological properties especially antimalarial, antibacterial, antifungisides, antiinflammation, antiallergic, antiproliferative, anticancer, antiparasitic and antibiocides.24-29 They also have antiseptic, antipyretic and antiperiodic properties. They are also used in dyes, rubber chemicals, and flavoring agents. Quinolines are very much used in transition-metal complex catalyst chemistry for uniform polymerization and luminescence chemistry.
The overall aim of this research was to investigate possible new antimalarial agents based on thiophene containing quinoline heterocyclic derivatives. Preliminary studies on some of these compounds carried out at the Department of Microbiology (Orchid Research Laboratories Ltd, NDD, Chennai, India) have shown that they possess moderate to potent antibacterial activity.30-32. There have been no literature reports of chemical structures of this type having any such activity. In view of the fact that some antibacterial agents also show anti-malarial activity, it was thus decided to explore the potential anti-malarial activity of compounds of this general type.
In the present study, we have synthesized a series of novel thienyl acrylate compounds with various heterocyclic moieties like quinoline, quinolinone, pyridine, pyrimidine, imidazole, thiazole, triazole, tetrazole and coumarins, which are known to posses biological activity. A basic study on the selected compounds of thiophene containing quinoline heterocylci derivatives biological activity with respect to larvicidal activity against malaria was carried out and the results are discussed.
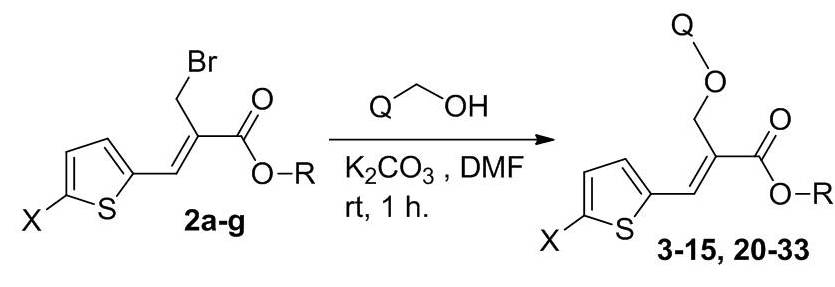
Scheme 1. Synthetic protocol of oxygen bridged thienyl acrylate compounds.
A series of oxygen bridged thienyl esters (3-15, 20-33) were synthesized by reacting the substituted bromo ester 2a-g with various hydroxyl heterocyclic compounds in the presence of K2CO3 and dry DMF (dimethylformamide) (Scheme 1). Completion of the reaction was confirmed by thin layer chromatography (TLC) and the crude products were isolated by simple precipitation in water. The resulted crude products were further purified by recrystallization method using methanol. Compounds 3-15 & 20-33 were prepared as per the Scheme 1 and their structures are depicted in Table S1 (see supporting information).31,32 The intermediate compounds 1a-g and 2a-g were prepared by following the earlier reported procedure (Scheme S1, see supporting information).31,32 The compounds 16 & 17 were prepared by treating the compounds 3 & 6 respectively with 4N NaOH solution and methanol under stirring at room temperature for 15 h. After the completion of reaction (monitored by TLC), solvent was completely distilled out under vacuum and pH of the reaction mass was adjusted to 6-7 using 10% dilute HCl (Scheme S2, see supporting information). The separated solid was stirred for 30 min., filtered and purified in methanol. The compounds 18 and 19 were prepared by condensation of compound 17 with benzyl amine/n-butyl amine in the presence of HOBt, EDC.HCl and triethyl amine in dry DMF were stirred for 10 h at room temperature (Scheme S3, see supporting information). After completion of reaction (checked TLC) water was added to the reaction mixture and stirred at room temperature for 30 min. The separated solid was filtered and purified in methanol. All the synthesized compounds were confirmed by spectral characterization using FT-IR, 1H NMR, 13C NMR and mass data.31,32
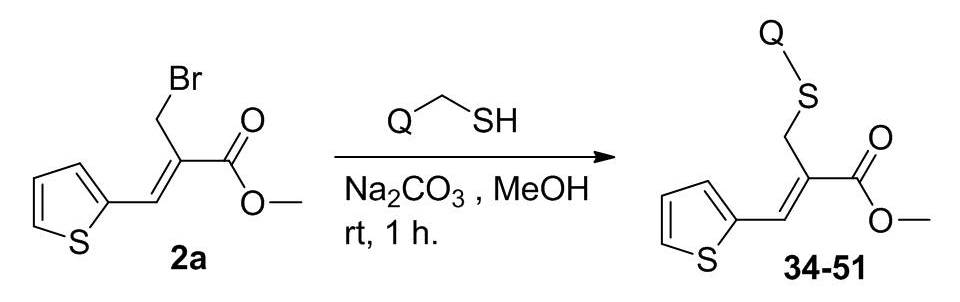
Scheme 2. Synthetic protocol of sulphur bridged thienyl acrylate compounds.
Sulphur bridged thienyl acrylate derivatives (34-51) were synthesized by reacting the bromo ester 2a with various thiol substituted heterocyclic compounds in the presence of Na2CO3 and methanol at room temperature (Scheme 2).30 Completion of the reaction was confirmed by TLC and the crude products were isolated by simple precipitation in water. The resulted crude products were further purified by recrystallization method using methanol. Compounds 34-51 were prepared as per the Scheme 2 and their structures are depicted in Table S2 (see supporting information). All the synthesized compounds were confirmed by spectral characterization using FT-IR, 1H NMR, 13C NMR and mass data.30
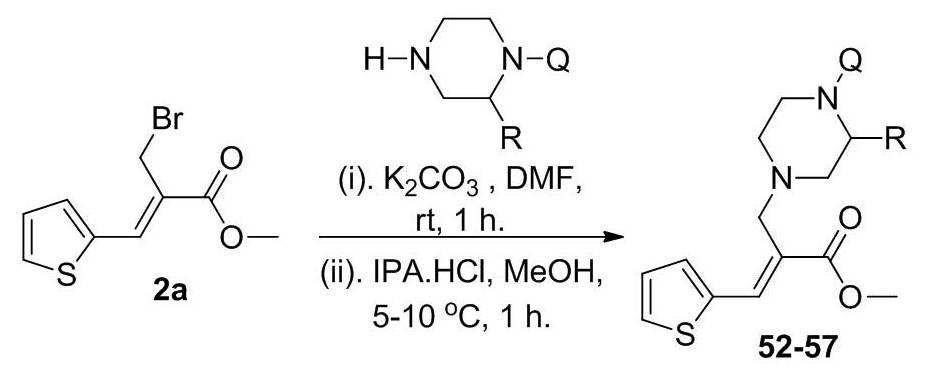
Scheme 3. Synthetic protocol of nitrogen bridged thienyl acrylate compounds.
A series of nitrogen bridged quinoline compounds (52-57) were synthesized by reacting bromo ester 2a with the piperazine substituted quinolinone compounds (I-VI) in presence of K2CO3 and DMF followed by addition of hydrochloric acid (Scheme 3). The compounds (I-VI) were prepared by the condensation of substituted piperazine and different quinolinone compounds in presence of DMSO at 80 °C for 3 h (Scheme S4, see supporting information). Completion of the reaction was confirmed by TLC and the crude products were isolated by simple precipitation in water. The resulted crude products were converted into hydrochloride salt by treating with isopropyl alcohol hydrochloride at room temperature in methanol to get pure compounds. Compounds 52-57 were prepared as per the Scheme 3 and their structures are depicted in Table S3 (see supporting information). All the synthesized compounds were confirmed by spectral characterization using FT-IR, NMR and mass data (see supporting information).
Selective compounds of thienyl acrylates containing quinoline substructures were studied for larvicidal activity against the second and fourth instar larvae of Cx. quinquefasciatus and Ae. aegypti. The results showed promising larvicidal activity against the second and fourth instar larvae of Cx. quinquefasciatus and Ae. aegypti. The toxic effect of synthesized compounds on the second and fourth instar larvae of Cx. quinquefasciatus and Ae. aegypti was tested and statistical data regarding LC50, LC90 were calculated and presented in Table 1 & 2.
Table 1. Larvicidal activity of compounds against second and fourth instar larvae of Culex quinquefasciatus.
|
Compound |
Culex quinquefasciatus |
|||
|
Second Instar Larvae |
Fourth Instar Larvae |
|||
|
LC50 |
LC90 |
LC50 |
LC90 |
|
|
3 |
35.89 |
87.65 |
70.89 |
158.56 |
|
6 |
30.50 |
70.12 |
61.25 |
110.16 |
|
9 |
35.86 |
76.89 |
98.55 |
170.25 |
|
10 |
37.25 |
76.45 |
74.12 |
144.36 |
|
11 |
49.46 |
99.25 |
76.24 |
143.56 |
|
12 |
24.21 |
49.54 |
47.88 |
95.36 |
|
13 |
25.05 |
50.22 |
49.55 |
94.26 |
|
14 |
24.56 |
49.31 |
48.88 |
96.34 |
|
15 |
28.46 |
56.65 |
55.85 |
108.18 |
|
17 |
38.42 |
76.98 |
64.56 |
148.25 |
Cx. quinquefasciatus and Ae. aegypti larvae were collected from various places with clean stagnant water. They were colonized and maintained continuously for generations in the laboratory free of exposure to pathogens, insecticides or repellents. They were maintained at 27±2°C, 75-85% RH with 14:10 L/D photoperiod cycle. The larvae were fed with dog biscuits, yeast extract in the ratio of 3:1. Water was changed everyday to avoid scum formation, which might create toxicity. Pupae were transferred from the trays to a cup containing tap water and placed in screened cages (45x45x45 cm dimension) where the adults emerged. The adult mosquitoes were reared in the glass cages of 45x45x45 cm dimension. Adults were continuously provided with 10% sucrose solution in a petridish with cotton wick. On the day of post-emergence the adult females were deprived of sucrose from 12 h and then provided with a mouse placed in resting cages overnight for blood feeding. After three days ovitrap was kept into the cages and the eggs were collected and transferred to enamel trays. They were maintained at the same condition for experiment.
Chloro quinoline and nitro quinoline substituted thienyl acrylate compounds showed very good larvicidal activity against the second instar larvae of Cx. quinquefasciatus and Ae. Aegypti compared to other quinoline compounds. The LC50 values of the tested compounds (3, 6, 9-15 and 17) against the second instar larvae were 35.89, 30.50, 35.86, 37.25, 49.46, 24.21, 24.56, 28.46, 25.05, and 38.42 ppm on Cx. quinquefasciatus (Table 1).
Table 2. Larvicidal activity of compounds against second and fourth instar larvae of Aedes aegypti.
|
Compound |
Aedes aegypti |
|||
|
Second Instar Larvae |
Fourth Instar Larvae |
|||
|
LC50 |
LC90 |
LC50 |
LC90 |
|
|
3 |
32.93 |
65.64 |
71.79 |
141.56 |
|
6 |
36.47 |
74.15 |
56.45 |
108.18 |
|
9 |
36.78 |
77.46 |
99.25 |
180.65 |
|
10 |
38.12 |
76.45 |
75.34 |
150.36 |
|
11 |
39.35 |
78.89 |
77.36 |
154.56 |
|
12 |
20.22 |
41.46 |
38.76 |
96.34 |
|
13 |
25.85 |
50.22 |
50.65 |
104.36 |
|
14 |
23.44 |
47.36 |
44.64 |
95.36 |
|
15 |
28.62 |
57.54 |
52.36 |
104.26 |
|
17 |
49.46 |
99.25 |
68.65 |
138.65 |
In Ae. aegypti the LC50 values of the tested compounds (3, 6, 9-15 and 17) against the second instar larvae were 32.93, 36.47, 36.78, 38.12, 39.35, 20.22, 23.44, 28.46, 25.85 and 49.46 ppm (Table 2). Highest larvicidal effect was observed in compound 12 LC50 (24.21 & 20.22) followed by compound 14 (24.56 & 23.44), compound 13 (25.05 & 25.85) and compound 15 (28.46 & 28.62) against the second instar larvae of Cx. quinquefasciatus and Ae. Aegypti. Among the ten compounds tested in this study, compound 12 was the most active and showed dose dependent mortality on mosquito larvae.
Conclusions
All the time there is a substantial demand for new and efficient processes in synthesizing novel heterocycles. The main focus of this research was to synthesize a new thienyl acrylate derivatives bearing different heterocycles and evaluation of anti-malarial activity of the synthesized derivatives. Structure of synthesized compounds were confirmed by the spectral characterization using analytical data such as FT-IR, 1H NMR, 13C NMR and mass spectra. The heterocyclic compounds with thienyl and quinoline type linkages have shown good larvicidal activity against the second and fourth instar larvae of Culex quinquefasciatus and Aedes aegypti.
Acknowledgements
Authors are thankful to the management of Orchid Chemicals and Pharmaceutical Ltd for the excellent support to perform this research project. We are also thankful for the kind support from the Staff of Orchid Chemicals and Pharmaceuticals Ltd in recording the spectral data and studying the biological activity of the synthesized compounds.
Notes and References
* Corresponding Author Details: Anand Sivadas, Department of Synthetic Chemistry, Orchid Chemicals and Pharmaceutical Ltd., Research and Development, Shollinganallur, Chennai, Tamilnadu, India.
E-mail id.: This email address is being protected from spambots. You need JavaScript enabled to view it.
† Supporting Information (SI) available: [Experimental Procedures, Spectral data].
1. Y. W. Chin, M. J. Balunas, H. B. Chai and A.D. Kinghorn, AAPS J., 2006, 8, 239.
2. F. E. Koehn and G. T. Carter, Nat. Rev. Drug Discov., 2005, 4, 206.
3. G. A. Cordell, M. L. Quinn-Beattie and N. R. Farnsworth, Phytother. Res., 2001, 15, 183.
4. E. H. Hughes and J. V. Shanks, Metab. Eng., 2002, 4, 41.
5. S. R. Norman, Drug Development Res., 2008, 69, 15.
6. R. Dahm, Human Genetics, 2008, 122, 565.
7. J. D. Watson and F. H. Crick, Nature, 1953, 171, 737.
8. National Academy of Sciences. Institute of Medicine. Food and Nutrition Board., Chapter 9 - Vitamin B . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B, Folate, Vitamin B, Pantothenic Acid, Biotin and Choline, (National Academy Press: Washington, D. C.), 1998, p. 346.
9. M. B. Davies, J. Austin and D. A. Partridge, Vitamin C: Its Chemistry and Biochemistry, (The Royal Society of Chemistry), 1991, p. 48.
10. H. M. Evans, O. H. Emerson and G. A. Emerson, J. Biol. Chem., 1936, 113, 319.
11. P. Furst and P. Stehle, J. Nutrition, 2004, 134, 1558S.
12. M. F. Perutz, Brookhaven symposia in Biol., 1960, 13, 165.
13. P. W. Brian,. Proceedings of the Royal Society of London. Series B, Biological Sci., 1978, 200, 231.
14. D. Rajiv, S. Suman, S. K. Sonwane and S. K. Srivastava, Adv. Biological Res., 2011, 5, 120.
15. M. G. Valverde and T. Torroba, Molecules, 2005, 10, 318.
16. B. C. Widemann, F. M. Balis, K. S. Godwin, C. McCully and P. C. Adamson, Cancer Chemo. Pharma., 1999, 44, 439.
17. https://en.wikipedia.org/wiki/Lornoxicam
18. https://en.wikipedia.org/wiki/Tenoxicam
19. A. J. Garton, A. P. Crew, M. Franklin, A. R. Cooke, G. M. Wynne, L. Castaldo, J. Kahler, S. L. Winski, A. Franks, E. N. Brown, M. A. Bittner, J. F. Keily, P. Briner, C. Hidden, M. C. Srebernak, C. Pirrit, M. O'Connor, A. Chan, B. Vulevic, D. Henninger, K. Hart, R. Sennello, A. H. Li, T. Zhang, F. Richardson, D. L. Emerson, A. L. Castelhano, L. D. Arnold and N. W. Gibson, Cancer Res., 2006, 66, 1015.
20. T. A. Yap, H. T. Arkenau, D. R. Camidge, S. George, N. J. Serkova, S. J. Gwyther, J. L. Spratlin, R. Lal, J. Spicer, N. M. Desouza, M. O. Leach, J. Chick, S. Poondru, R. Boinpally, R. Gedrich, K. Brock, A. Stephens, S. G. Eckhardt, S. B. Kaye, G. Demetri and M. Scurr, Clin. Cancer Res., 2013, 19, 909.
21. http://www.drugbank.ca/drugs/DB01600
22. http://www.drugbank.ca/drugs/DB00870
23. I. M. Opsenica, T. Z. Verbić, M. Tot, R. J. Sciotti, B. S. Pybus, O. Djurković-Djaković, K. Slavić and B. A. Šolaja. Bioorg. Med. Chem., 2015, 23, 2176.
24. H. Cairns, D. Cox, K. J. Gould, A. H. Ingal and J. Suschitzky, J. Med. Chem., 1985, 28, 1832.
25. R. Jain, S. Jain, R. C. Gupta, N. Anand, G. P. Dutta and S. K. Puri, Indian. J. chem., 1994, 33B, 251.
26. A. Mohammed, N. Abdel-Hamid, F. Maher and A. Farghaly, Czech. Chem. Commun., 1992, 57, 1547.
27. M. Croisy-Delcey, A. Coroisy, D. Carrez, C. Huel, A. Chiaroni, P. Ducrot, E. Bisabni, L. Jin and G. Leclercq, Bioorg. Med. Chem., 2000, 8, 2629.
28. A. Dlugosz and D. Dus, Farmaco, 1996, 51, 367.
29. A. H. Abadi and R. Brun, Arzneimforsch. Drugs. Res, 2003, 53, 655.
30. A. Sivadas, M. Paul-Satyaseela, T. Bharani, U. S. Kumar and N. Subbaraya, Int. J. Pharm. Sci. Res., 2011, 2, 27.
31. A. Sivadas, M. Paul-Satyaseela, T. Bharani, U. S. Kumar and N. Subbaraya, Int. J. Pharm. Sci. Res., 2011, 2, 893.
32. A. Sivadas and N. Subbaraya, Int. J. Pharm. Sci. Res., 2011, 2, 1007.
